drug development, and clinical trial drugs
GREEN8 can accurately respond to the rapidly growing international logistics demands in bio-pharmaceutical industry and advanced scientific research field, which are becoming increasingly multinational and borderless. Through its flawless reputation in Japan, GREEN8 provides specialized solution to ensure a smooth and reliable logistics in cell and gene research, regenerative medicine, cross-border joint research for new drug development, and clinical trials projects, which requires a seamless solution for handling bio-pharma products that requires complex import, export regulations and license requirements, as well as temperature control and traceability requirements.
GREEN8 is your partner in Japan for providing a specialized and reliable door-to door service in biopharma and healthcare sectors.
Fast and secure shipping service
・International express transportation by fastest and most reliable routes
・GREEN8 make sure your bio-pharma products are delivered on time, without delay.
・On-time collection of patient samples from study sites and laboratories globally
100% Tailor-made Logistics Solution
・Bilingual Japanese and English personnel assigned to each project based on client requirement
・White-glove service for biological specimen and clinical trial specimens
・Packing and re-labeling of clinical trial drugs as well as disposal of biological specimens
・Dedicated local staff conducting on-site inspections and preliminary confirmation of each requirement
・Corrective and Preventive Actions and guidelines in place to ship and distribute bio-pharmaceutical
・Customized pickup and delivery service for hospitals, laboratories, universities, and blood centers
・Japan local coordination with medical sites and clinical supply centers from pre-trial set-up to execution stage
・24/7 pickup and delivery of biological samples, infectious material, and clinical trial kits
・Distribution of investigational drugs from storage sites to clinical trial participant
GREEN8 biopharma Logistics serves life science business from Japan
・Transportation of bio-pharmaceutical material while conducting clinical trials internationally or in multiple countries, including Japan
・Knowledge and experience in dealing with laws and regulations under the jurisdiction of the Ministry of Health, Labor and Welfare and other government agencies and in applying for import/export licenses
・Pre-trial logistics consultation service including legal compliance, regulations of competent government authorities, and site selection for implementation
・Highest level of care in management, storage, packaging, transportation scheduling, and route selection for clinical trial kits according to GDP standard
Fast and secure shipping service
・GREEN8 specializes in the logistics of life science related products such as pharmaceuticals, hazardous goods and medical devices, and are well versed in market trends in Japan.
・Validated packaging solutions for all payloads and temperature ranges along with appropriate pre-cooling procedures are in place in our Japan centers.
・Through years of logistical requests from academics, research institutes and medical centers, GREEN8 is confident in its accumulated know-how in distribution and transportation of biological and pharmaceutical material both domestically in Japan, and international shipments.
1
Full support from pickup to required documentation
2
Logistical customization tailored to your material contents and volume (transportation from a single tube to commercial volume of pharmaceutical product)
3
Keeping product integrity through validated packing solutions and transportation in specified temperature zones ( CRT, Refrigerated, Frozen, and Cryogenic)
4
Traceability in clinical trials through preparing and submitting analysis and evaluation reports (temperature recording in 5-15 minute increments)
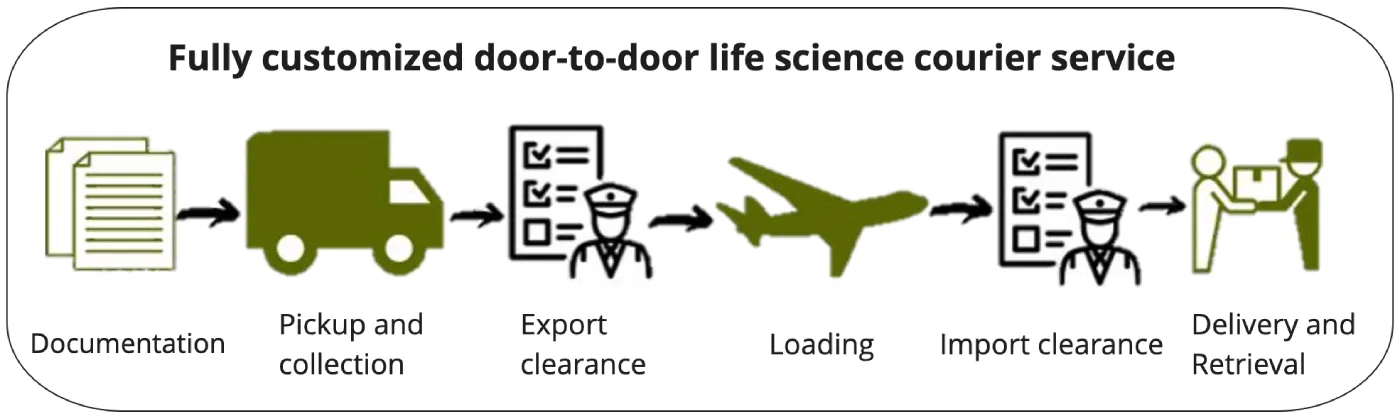
GREEN8 offers complete customized arrangement for each logistical needs of the clients. We provide one-stop, precise service from assistance in documentation and confirmation of local regulations, to acceptance of the material, air freight, custom clearance, and delivery.
GREEN8 will conduct regulatory surveys and prepare documents on behalf of clients as required, depending on the products and destination. Our dedicated staff will collect materials at designated site and we will deliver it anywhere in the world with safest and most reliable route. GREEN8’s white glove service will prepare for validated packing solutions in which clients have no worries to precool packing solutions in advance. GREEN8’s dedicated trained staff will support you in tracking and handling your material all the way from collection to delivery.
Designated temperature range
・Refrigerated: 0-9°C / 2-8°C / 5-10°C
・Frozen: 0 to -20℃
・Deep Frozen: -60℃ to -80°C
・Cryogenic: -150°C to -196°C
Optional temperature-controlled services
・Temperature Data Analysis
・Provision of special cargo insurance (includes special temperature clause)
From clinical trial drugs and new drug development samples to research precision equipment, GREEN8 safely and reliably deliver many shipments related to the medical and pharmaceutical industries. We maintain real-time monitoring and tracking of cargo status 24 hours a day, 365 days a year under strict temperature control, from CRT to refrigerated and frozen temperatures.
GREEN8 is also capable of handling special packaging solutions for shipments containing hazardous or infectious materials. Compliance and transparency are important factors for GREEN8 and our client, and hence so we operate through our GDP-compliant facility and procedures in which are suitable for integrated logistical service required for temperature sensitive materials such as pharmaceutical raw materials, APIs and investigational drugs.
GREEN8 actively transport human-derived, animal- and plant-derived cells. We can transport regenerative and reproductive medical cells, stem cells for regenerative medicine, oocytes, fertilized eggs for humans, as well as cells and embryos for animal at temperatures below -196℃ using validated containers and liquid nitrogen cryogenic tanks. GREEN8 professionals can also handle specimens containing infectious substances including Category A and Category B biological material, as well as other substances considered hazardous and dangerous in Japan.
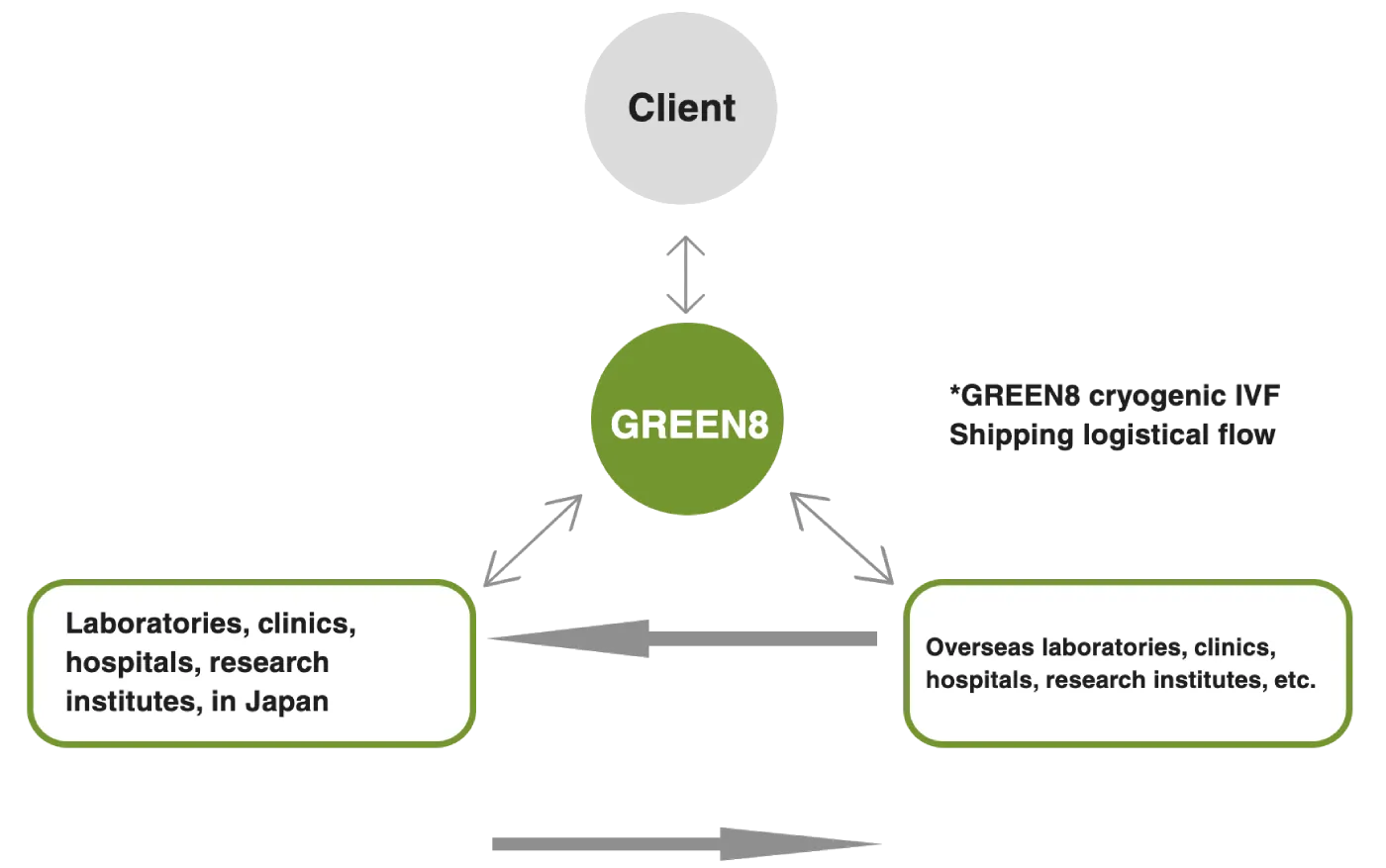
IVF Shipping Solutions
GREEN8 IVF Shipping Solutions provide a total service for the transportation of frozen sperm, eggs, and fertilized eggs in Japan, as well as international transportation of cryogenic IVF material.
GREEN8 has been providing international transportation services for overseas fertility treatment since 2013, and we have been performing more than 200 international shipments per year. We arrange one dedicated dry vapor shipper per customer and transport previous reproductive materials with the utmost care.
From pickup to delivery, a dedicated Green8 representative provides status for each process and 24-hour online tracking service is available. Green8 IVF cryogenic logistics is arranged through chartered collection and delivery from each IVF and medical clinic, and our representative will handle all essential international shipping documentation, customs, and clearance procedures, and liaise with airport security to ensure a smooth and safe transfer. Based on client’s treatment schedule, Green8 coordinates with each clinic to propose an optimal logistical schedule.


For the transportation of living organisms, GREEN8 provide reliable and speedy transportation services based on our long-standing expertise in the transportation of live lab animals, including SPF mouse, rats and rabbits, as well as other small laboratory animals. We are proud of supporting our clients to improve their operational efficiency by investigating import and export regulations for each of the live animal for specific destination country, animal quarantine, and other complicated regulatory procedures.Green8’s trained and professional staff can handle the preparation of documents and application documentation to meet import and export permits and license requirements, and required pre- confirmation with regulatory bodies in advance.
Implementation of cross-boarder clinical trials require expertise in logistical coordination as well as regulatory compliance of investigational drugs and patient specimens. GREEN8 is confident in its experience from Japan to conduct logistical support for clinical trials in Japan as well as international logistics for demanding global clinical trials. Unlike conventional logistics providers and general courier services, which transport clinical trial drugs as part of a wide range of general freight forwarding services, GREEN8 offers an all-in-one service as a specialized carrier, from the pre-implementation stage to the post-implementation collection of patient samples for clinical trials. GREEN8 provides an all-in-one service from the pre-trial preparation stage to post-trial patient sample collection.
There are many items that are not generally considered dangerous goods, such as flammable, corrosive, poisonous, or oxidizing materials, but are treated as dangerous goods during transportation. These items are subject to rigid packaging requirements and documentation in accordance with the “Recommendations for the Transport of Dangerous Goods” (commonly known as the “Orange Book”) established by the United Nations.
The Orange Book contains number of rules and regulations, such as the “UN number and name to be used for the carriage of dangerous goods,” “labels to be displayed for each class,” “UN classification,” and “requirements for containers for transport (packaging and packing methods). In some cases, the same dangerous goods may be loaded or unloaded by sea or air, and the regulations differ, requiring complicated checks.
GREEN8 specialists in handling dangerous goods will prepare the necessary documents and investigate the most appropriate packing solution for each product, and then transportation of these materials respectively.
Temperature Control Logistics x Hazardous Materials x GDP Compliance
GREEN8 provides storage services for bio-pharmaceutical products with the added value of GDP compliance in respect to low-temperature requirements as well as explosion-proof specifications for DG products, which are still rare in Japan. We provide a supply chain solution for the pharmaceutical manufacturing industry in Japan.
Based on our track record of GDP-compliant transportation of pharmaceuticals that require strict temperature control, Green8 realize smoother logistics of pharmaceuticals through both transportation and storages solutions.
IATA Certified Validated Passive Containers

IATA certified Cryogenic Dry Vapor Shippers

IATA certified Cryogenic Dry Vapor Shippers
GREEN8 use IATA-certified dry shipper containers that maintain stable cryogenic temperatures during international and domestic transportation. The dry vapor tanks we use are capable of maintaining temperatures of -150℃ to -190℃ for more than 15 days, thanks to the absorption of liquid nitrogen by the sponge built into these dry vapor tanks.
Lease and Rental service
GREEN8 Japan also provides rental of dry vapor shippers as well as the carry-case containers. Pre-cooling with liquid nitrogen is also available through our LN2 depos across Japan. For more information on rental and lease details, please contact us at by email (info@green8.co.jp) or telephone us ( +81-3-6206-2657) or contact us through online inquiry form.

GREEN8 offers a variety of temperature monitors to meet various demands for transporting bio-pharmaceutical materials, chemicals, and biological specimens. Temperature monitors are available as in single-use or multi-use loggers, humidity sensors, or other measurement loggers. Range of loggers are available from cryogenic ultra-low temperature of -200℃ to high temperature of +100℃, providing the right solution for any type of supply chain solution.
Data analysis and reporting service is also available.